Randomised controlled trial to examine the effect of health-related press releases on news coverage.
Study aim:
The aim of this study (funded by the ESRC transformative scheme) is to run a randomised controlled trial to see whether and how wording of press releases influences news coverage for health-related research.
By collaborating with press offices around the UK we will be able to build a rich evidence base of ‘what works’ in terms of accuracy, prominence and quantity of news coverage. This database can then be used to guide evidence-based policy.
Why is this important?
Our recent research (Sumner et al., 2014, BMJ) suggests that up to 40% of health-related press releases contain exaggerated statements relative to the associated journal article (based on 462 health and biomedical press releases issued by the 20 Russell Group Universities in 2011).
Furthermore, we found similar levels of exaggeration between the press releases and the news articles that followed – such that when the press release contained exaggerated statements, the news articles were up to 56 times more likely to also be exaggerated. Our findings suggest that the majority of exaggeration in the news is already present in the preceding press release and, surprisingly, does not occur de novo in the media. Contrary to assumption, we also found no evidence that press releases containing exaggerated statements were more likely to attract news uptake or result in more news articles than those which did not contain exaggerated statements.
Such exaggeration has widespread implications for public health as mainstream news is still the primary means through which the public obtain information on science and health. Although accurate claims in the media are not sufficient for the public to make well-informed decisions about health, we argue that they are the necessary starting point and our results suggest that the scientific community has the ability to improve this situation through their press releases.
Method:
The study is targeting only press releases based on peer-reviewed research of relevance to human health.
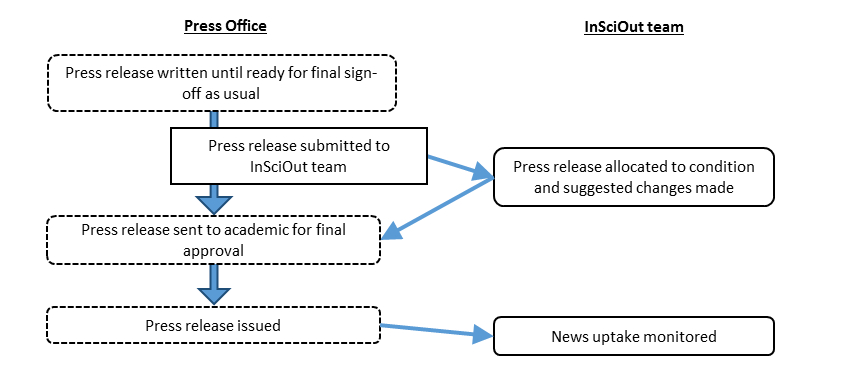
Press officers and academic authors will write press releases, in the usual way, until the press release is ready for final approval by the authors and interested partners (i.e. collaborators and funding bodies). At this stage, the eligible press releases will be sent to the InSciOut team and randomly assigned to have suggested modifications or not.
The suggested modifications will be sent back by email to the press office within pre-agreed timelines. Suggested modifications will depend on the type of study as described in the journal article itself. The press office and authors retain final say on all press release text as usual.
The InSciOut team will then monitor the news articles that follow.
If you are an academic author interested in participating in the trial please see below for a list of frequently asked questions or please email us if you have any of your own: insciout@cardiff.ac.uk
Frequently asked questions
What can be gained from the trial?
This trial will be the first of its kind, investigating the causal relationship between wording of the press release and news coverage. We will end up with a rich database that can inform evidence-based practice. As well as looking at how wording affects accurate news coverage, we can also investigate how other factors of interest affect news uptake e.g. day of release, embargo period. There may also be benefit for the sector working together to improve practice, if improvement is possible (or else we’ll produce evidence that practice is already optimal).
What are the risks of the trial?
Although our previous research found no association between subtle exaggerations and news uptake, there is always the possibility that the changes we suggest could reduce news uptake. We will examine evidence for this possibility as we go; if evidence builds that a condition is harmful to uptake (according to criteria pre-agreed with the press officers), we will stop using it.
What will be the suggested changes to the press release?
The suggested changes will be based on the associated journal article and will be small changes to the wording and phrasing. For the integrity of the trial is it important that academic authors remain blind to the experimental conditions, however, authors and press officers will always have final approval for the press release. Suggested changes will be presented as ‘tracked changes’ and can easily be accepted or rejected.
How long is the trial for and how big will it be?
The trial will run for approximately 12 months. We are aiming for 500-1000 press releases across many press offices. The greater the number of press releases, the more powerful will be the trial.
If you have any questions regarding the trial please contact us:
tel: 029 2087 0708
email: insciout@cardiff.ac.uk

