WELCOME TO THE UK CROSS-LINKING CONSORTIUM WEBSITE
The UK Cross-linking Consortium was established in 2013 with funding from the Medical Research Council with the aims of:
- Establishing a code of best practice for corneal cross-linking in order to standardise the treatment and its measurement outcomes.
- Providing information and advice to national bodies about developments in corneal cross-linking.
- Providing a forum for ophthalmologists and vision scientists to develop research collaborations and coordinated multi-center studies.
Member Updates
2024 Joint British Society of Refractive Surgery and UK-Cross-linking Consortium Meeting: A big thank you to our speakers and Consortium members for making our first joint meeting with the British Society of Refractive Surgery such a success. The meeting took place in Oxford on 22nd June 2024, with over 80 ophthalmologists, optometrists and vision scientists attending the UK-CXL Consortium session to hear about the latest advancements in cross-linking research and participate in lively discussions about the topic. Click here to find out more.
Survey of Corneal Cross-linking Practice Patterns in the UK: Many thanks to all our Consortium members who contributed to a national survey of corneal cross-linking practice. The survey helped us to identify current trends in corneal cross-linking practice in the UK, as well as variations between centers. We hope that the publication of the survey findings in Eye will stimulate further discussion about best practice for corneal cross-linking and the monitoring of keratoconus patients. The full findings of this survey can be viewed here.
The future of corneal cross-linking in Wales: Following an evidence appraisal undertaken on behalf of the Welsh Government in January 2021, Health Technology Wales have concluded that ‘the evidence supports the routine adoption of corneal cross-linking (CXL) for children and adults with progressive keratoconus’ and recommend that NHS Wales should ‘adopt this guidance or justify why it has not been followed’. The contribution of the UK-CXL Consortium to the consultation process has been acknowledged in the updated Health Technology Wales Guidance [published May 2021].
Keralink Trial: The much anticipated results of the KERALINK Randomised Controlled Trial which involved 60 young patients have now been published. The study showed that participants receiving cross-linking plus standard care were less likely to demonstrate clinically significant keratoconus progression than those that received standard care alone. Click here to read the full publication and keep up-to-date with other current research relating to corneal cross-linking.
Development of a national corneal cross-linking register: A national corneal cross-linking register and keratoconus monitoring tool has been developed by the UK Cross-linking Consortium, with support from the The Royal College of Ophthalmologists’ Informatics and Audit Sub-committee. Following a successful trial at Moorfields Eye Hospital in 2017, the Keratoconus Module is now available to all users of OpenEyes v1.18/2.0 for monitoring patients before and after cross-linking. The data set will provide a basis for clinical care, outcome analysis, clinical audit, revalidation, and research.
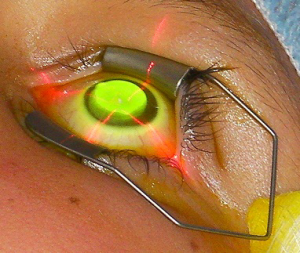 The potential of ultraviolet-A light (UVA) to cross-link tissues in the presence of the non-toxic photosensitising agent riboflavin had been known for some time, but it was not until 1998 that a group from Dresden suggested it as a potential therapeutic treatment to strengthen the corneal stroma….Read more
The potential of ultraviolet-A light (UVA) to cross-link tissues in the presence of the non-toxic photosensitising agent riboflavin had been known for some time, but it was not until 1998 that a group from Dresden suggested it as a potential therapeutic treatment to strengthen the corneal stroma….Read more
Cross-linking the cornea using riboflavin and ultraviolet A light has been widely adopted, refined and applied in a range of corneal surgeries and pathologies where the strength of the cornea might be compromised….Read more
Epithelium-off photochemical corneal collagen cross-linking has been approved for general use in the NHS. However, the National Institute for Health Care Excellence (NICE) encourages further research into the use of riboflavin/UVA cross-linking for keratoconus and keratectasia, especially epithelium-on (trans-epithelial) cross-linking and combination procedures. Click here to see the current NICE guidelines for epithelium-off and epithelium-on cross-linking.
Last updated: 02/12/2021