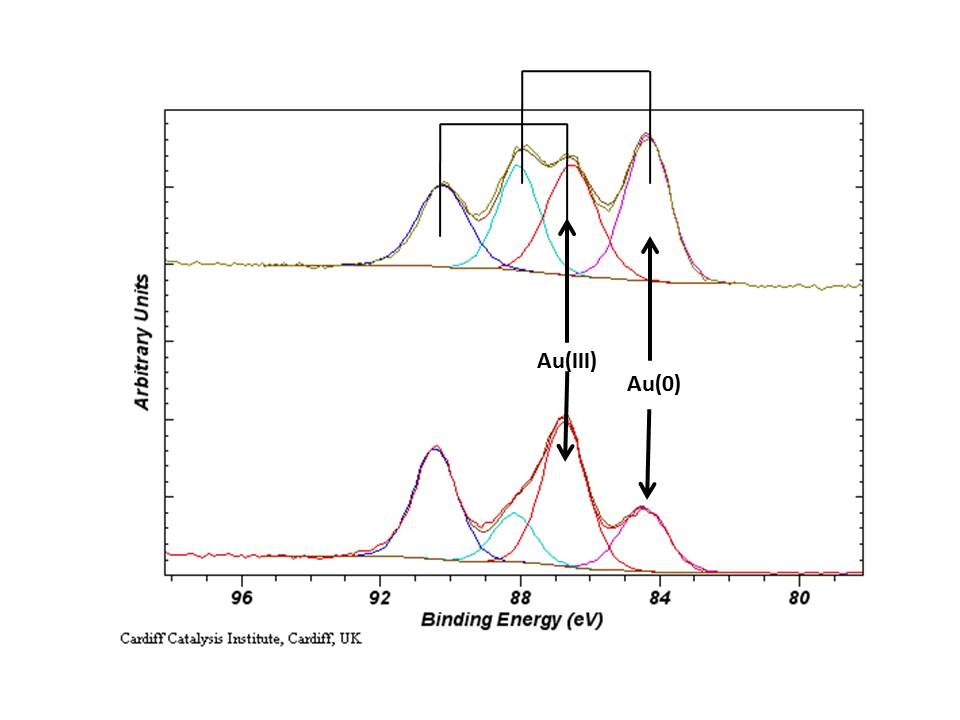
There is a lot of current interest in the chemistry and especially the catalysis of gold, therefore there is a great interest in the reliable assignment of metallic and ionic gold species in XPS. Typical Au(4f7/2) binding energies are given below:
Metallic Au: 84.0 eV
Au(I): ca. 85.0 eV
Au(III): ca. 86.0 eV
Spin-orbit splitting of Au(4f) peaks: 3.7 eV
Important notes:
For supported catalysts, depending on the support material, size of the Au nano-particles and treatment conditions, the binding energy of the Au(4f) peaks will shift. The influence of final and initial state effects should be considered in all cases. See for example Phys. Chem. Chem. Phys., 2003, 5, 172-177 and ACS Nano, 2012, 6 (8), pp 6600–6613.
Cationic gold, especially Au(III) species, undergo photo-reduction by the photo-emission process and therefore, the Au(4f) region should be acquired on its own, then again with the remaining regions of interest to assess the degree of reduction. An example is given below for the analysis of the Au(III) salt – HAuCl4.