Carbon, in its many forms may exhibit full sp2 or sp3 hybridisation, or a combination. Equally it may be bound to O, S, N, metals in different ratios or be present as a carbide phase. In fact the majority of samples exposed to the atmosphere will have a considerable concentration of carbon contamination ~ 2 nm thick.
Common carbon, C(1s) reference energies:
Adventitious Carbon: 284.8 eV (C-C and C-H bonds).
- Typically there will be some C-O and C=O structure associated (286 – 288 eV), however depending no the nature of the material, this is not always a good reference despite its wide use – for example carbide materials or for aluminium, the carbon is typically around 286 eV.
Organic/Polymeric materials (these are average values – See reference [1]):
- C-C/C-H ~ 285.0 eV
- C-O ~ 286.0 eV
- C=O ~ 288 – 289 eV
- CF2 ~ 292 eV
- CF3 ~ 293 eV
Inorganic carbon:
- Carbide ~282 – 283.5 eV
- Carbonates ~ 288 – 290 eV
HOPG/Graphite, Diamond, Graphene:
- SP2 carbon ~ 284.0 – 284.5 eV (HOPG typically seen at 284.5 eV )
- SP3 carbon ~ 284.5 – 285.0 eV
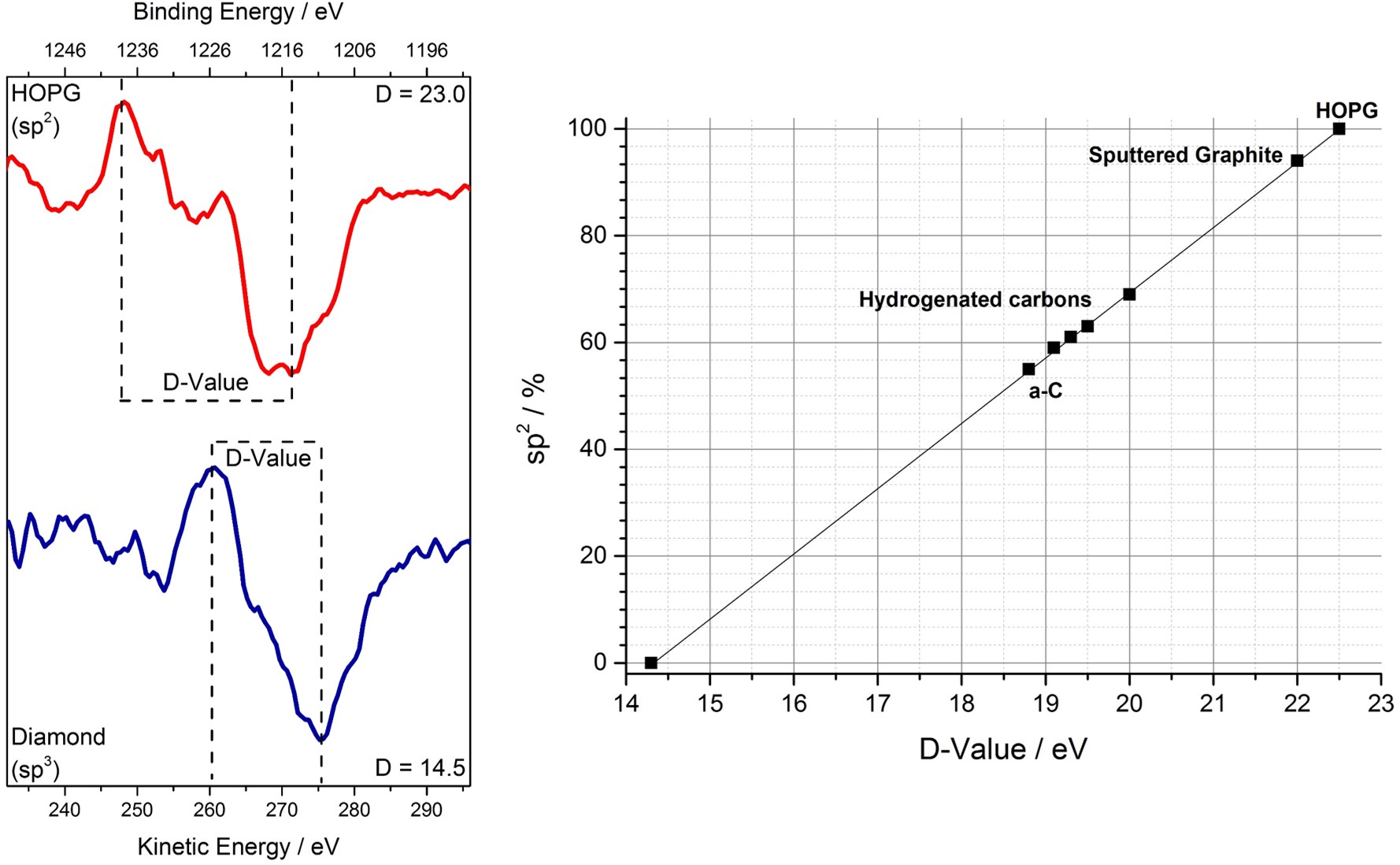
By far the best way of determining sp2/sp3 ratio is by means of the D-value [2]. By recording the carbon x-ray induced Auger peak and taking the maxima and minima from its differential form (figure 1), then the sp2% may be found.
References
[1] Beamson G, Briggs D. High Resolution XPS of Organic Polymers – The Scienta ESCA 300 Database. John Wiley & Sons: Chichester, 1992.
[2] J.C. Lascovich and S Scaglione, Appl. Surf. Sci. 78 (1994) 17-24

